Tuning electrochemically driven surface transformation in atomically flat LaNiO3 thin films for enhanced water electrolysis
2021-05-01 | Tomáš Duchoň
10.1038/s41563-020-00877-1
10.1038/s41563-020-00877-1
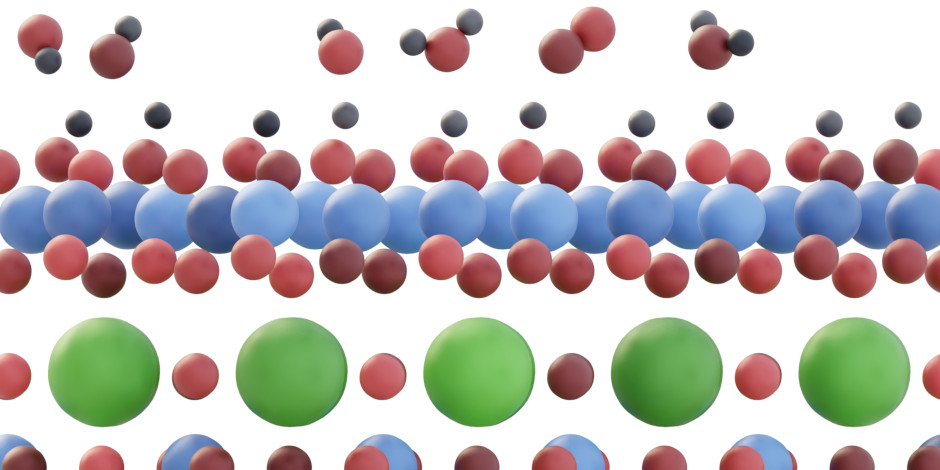
Surfaces of perovskite-type oxides often decompose when subjected to electrochemical driving forces. While the decomposition makes bulk properties inherent to the perovskite structure difficult to utilize in electrochemical conversion, the products of the decomposition can themselves exhibit remarkable activity. Such is the case of LaNiO3(001), where overpotential differences of up to 150 mV are observed for the oxygen evolution reaction (OER) depending on the terminating species (i.e., La or Ni). In our recent paper, we track the differences to the formation of stable, disordered NiOx/NiOOH layers on the Ni-terminated LaNiO3(001) surfaces under operation, with the transformation being thermodynamically inaccessible from the La-terminated LaNiO3(001) surfaces. Ni oxides and (oxy)hydroxides are well-known OER catalysts, which explains the activity divide between the two surfaces. Importantly, the underlying perovskite lattice plays a role in anchoring the electronically coupled surface phases, offering potential benefits with respect to the use of single-phase Ni oxides and (oxy)hydroxides to catalyze OER. The results are published in the current issue of Nature Materials.